The History of Reverse Osmosis
The History of Reverse Osmosis
Osmosis has taken place for millions of years and happens in our bodies every day.
Table of Contents
- What is Reverse Osmosis?
- When Was Osmosis Discovered?
- Reverse Osmosis Through the Years
- Common Reverse Osmosis Applications
When Was Osmosis Discovered?
The process of osmosis through semipermeable membranes was first observed in a laboratory setting in 1748 by Jean-Antoine Nollet, using a pig’s bladder as a membrane. He proved that a solvent could pass selectively through a semi-permeable membrane through the process of natural osmotic pressure and the solvent will continually enter through the cell membrane until dynamic equilibrium is reached on both sides of the bladder.
Reverse Osmosis Through the Years
Over the next 200 years, osmosis was only a phenomenon observed in the laboratory. In 1949, the University of California at Los Angeles was the first to look to osmosis and semipermeable membranes for a solution of removing salt from seawater. Researchers from both the University of California and the University of Florida successfully produced fresh water from seawater in the mid-1950s, however, the product was not commercially viable due to high flux. John Cadotte, of FilmTec Corporation, later discovered that membranes with particularly high flux and low salt passage could be made by interfacial polymerization of m-phenylenediamine and trimesoyl chloride. By 2001, over 15,00 desalination plants were up and running, or planned for, around the world.
In 1977, Cape Coral, Florida became the first municipality in the United States to use reverse osmosis on a large scale with an initial operating capacity of 3 million gallons per day. By 1985, due to the rapid growth in population of Cape Coral, the city had the largest low-pressure reverse-osmosis plant in the world, capable of producing 15 million gallons per day.
What is Reverse Osmosis?
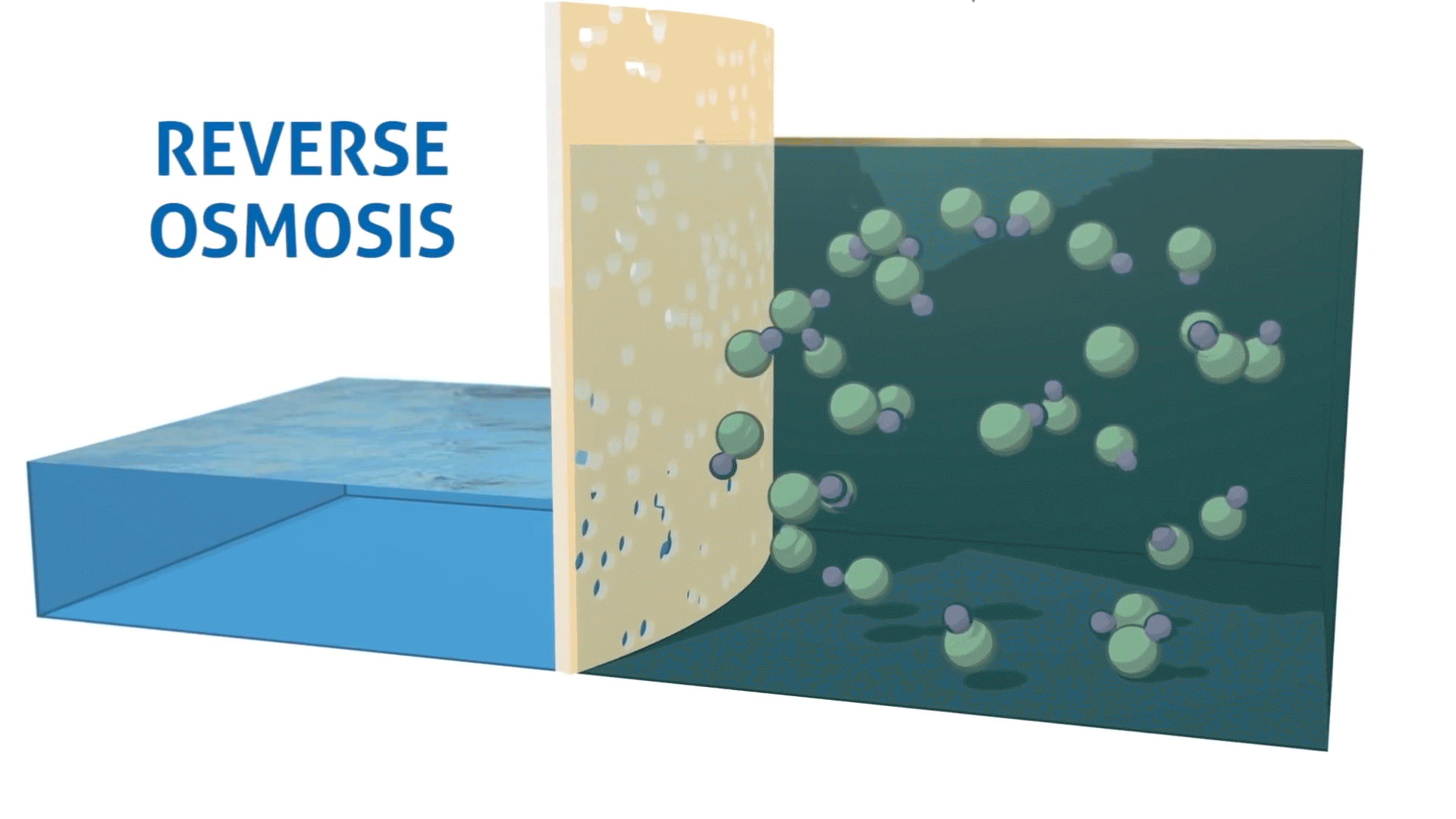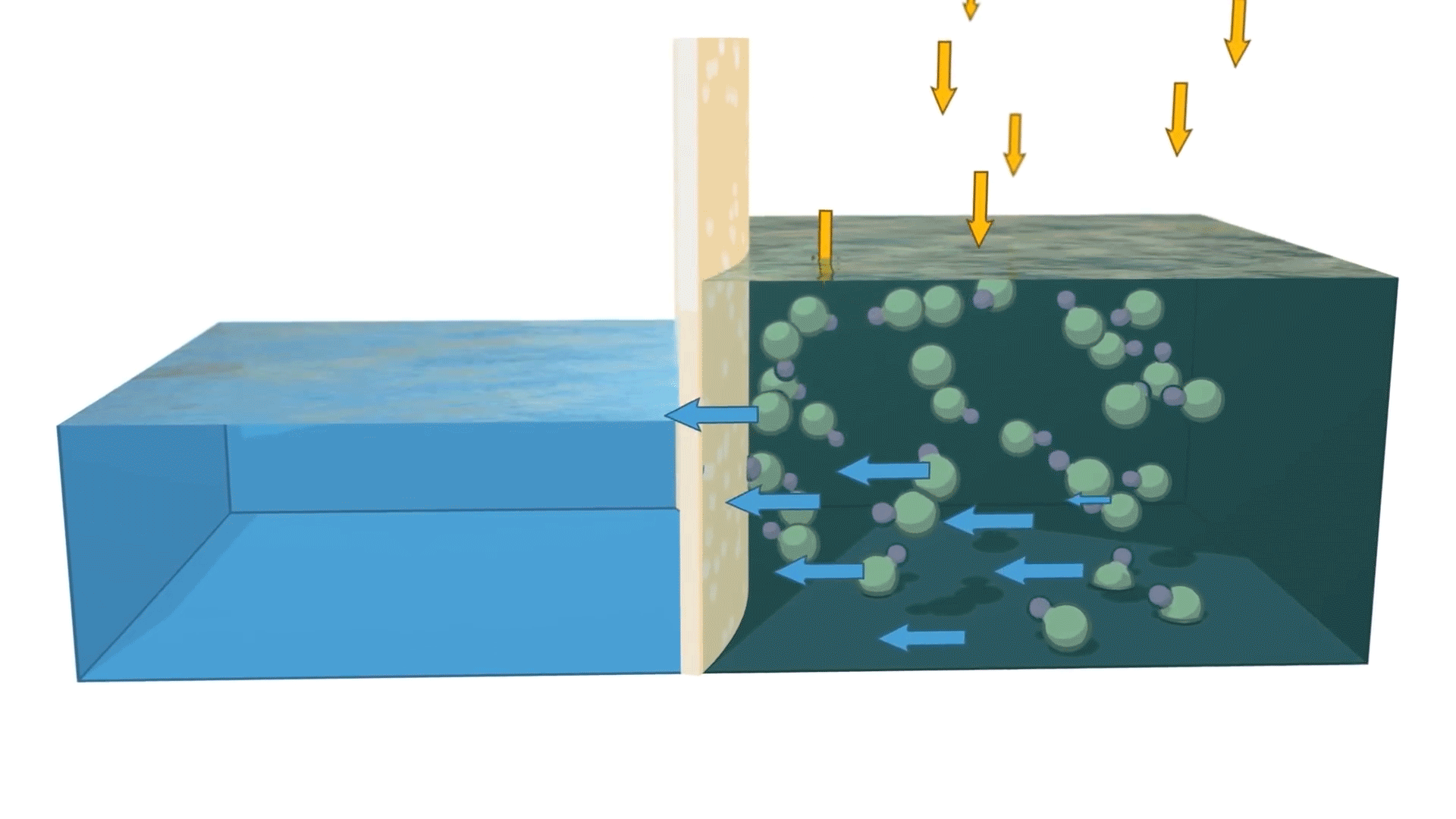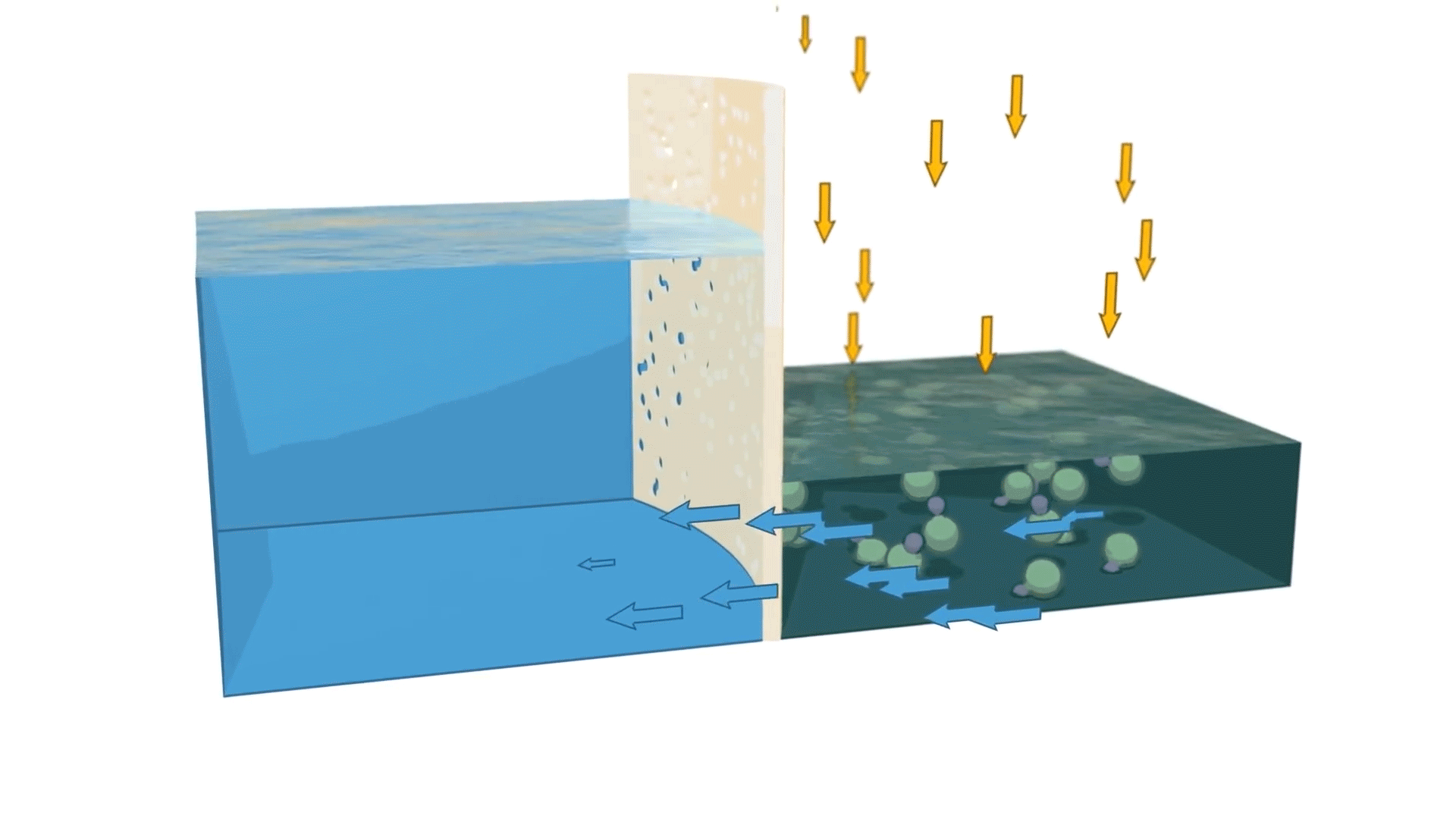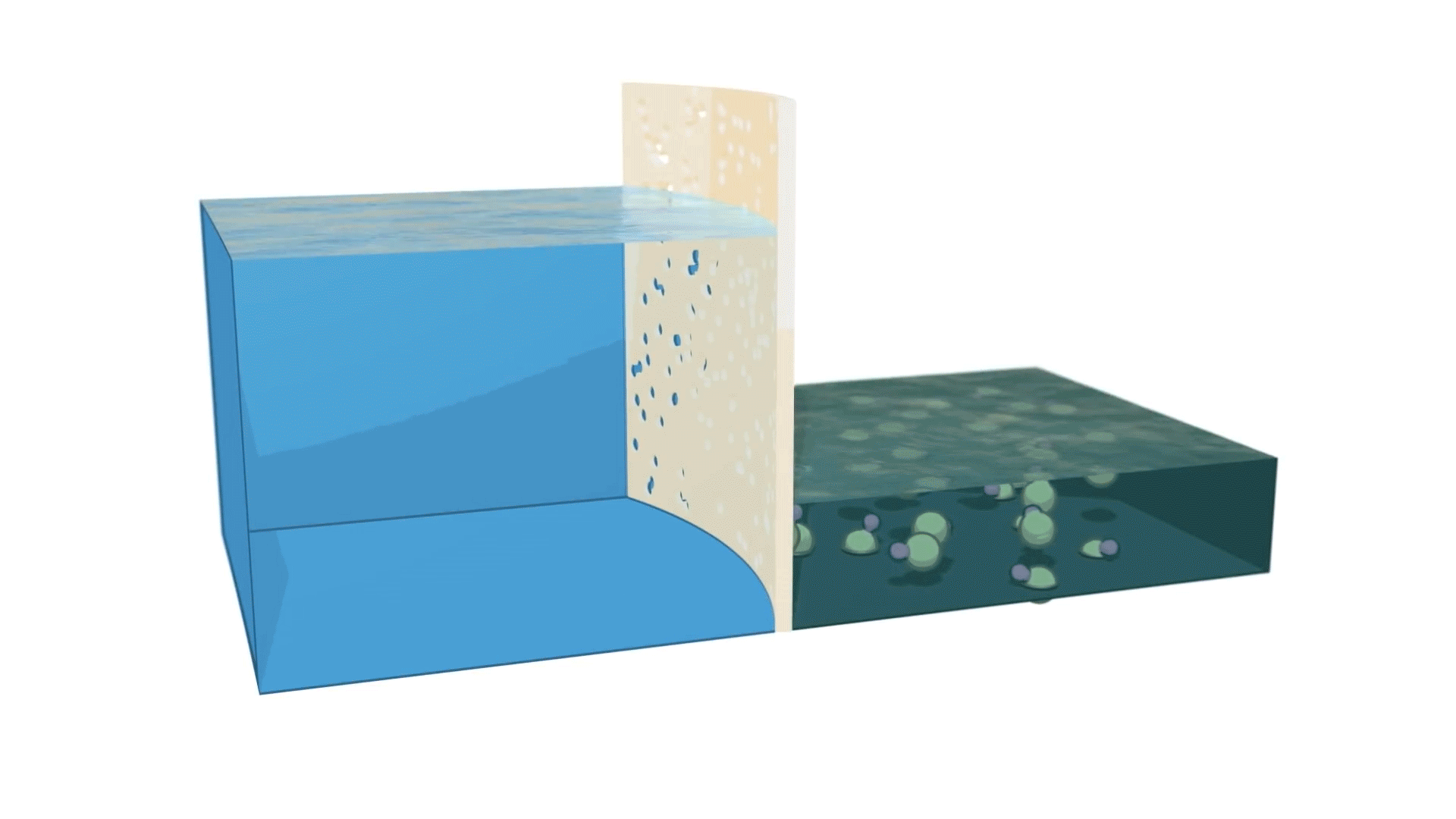
Reverse osmosis is the process of forcing a solvent from a region of high solute concentration through a semipermeable membrane to a region of low-solute concentration by applying a pressure in excess of the osmotic pressure. The largest and arguably most important application of reverse osmosis is the separation of pure water from seawater and other brackish waters: The seawater, or brackish water, is pressurized against one surface of the membrane, causing the transport of salt-depleted water across the membrane and creating pure water on the low-pressure side.
BOILER FEEDWATER
Industries that rely on steam as a driving force for turbines, an energy source for heating, or product processing know that suspended and dissolved solids in a steam generator case scale and can lead to tube failure. Poor boiler feedwater quality increases energy consumption, reduces steam quality and purity, and can reduce both production rates and product quality. Including reverse osmosis systems in the boiler feedwater makeup circuit to replace or pretreat for ion exchange increases production reliability and significantly reduces operating and maintenance costs.
ULTRAPURE WATER (MICROELECTRONICS AND PHARMACEUTICAL)
For microelectronics and pharmaceutical manufacturing, potable water supplied by a local municipality is not nearly pure enough. Their product specifications require water free of bacteria and organics, with dissolved solids levels that are 10,000 times lower than those found in potable drinking water. The first step in reducing the suspended solids levels is reverse osmosis where 98% of the influent water salts can be removed before additional treatment with advanced ion exchange or continuous deionization systems produce the ultrahigh purity water they need.
INGREDIENT WATER (FOOD & BEVERAGE)
High quality water is a critical ingredient in the food and beverage processing industry. Not only is it the obvious foundation of the bottled water industry but it is also important in beer production, where high quality water is used in the proofing process for alcohol beverage production. Meat and poultry processing as well as fresh pack and processed food production also need high quality water requirements. RO product water can meet all these needs. In addition to the ability to reduce dissolved solids, reverse osmosis can also remove pollutants and microorganisms.
WATER REUSE & RECYCLING
The assumption that an unlimited quantity of affordable raw water is available to industry is no longer valid. Manufacturers are looking for alternate water sources to assure operational reliability and reduce potable water consumption. Spent process streams and plant wastewater effluent are now considered as possible water supplies and reverse osmosis can be an important technology in reducing dissolved solids levels and enabling these water streams to be reused and recycled back into the plant.
DESALINATION
The earth is covered primarily by water, however, much of this water has a high salt content and cannot be used for either human consumption or industrial processing. However, in certain regions, the only available water source is either brackish water or seawater. Reverse osmosis systems can reduce the TDS level of brackish water and seawater to potable levels and further treatment can generate the higher purity waters needed for industry, medical care, and institutional use.