Control of Odors in the Brewing & Food Processing Industries – Part 2
Control of Odors in the Brewing &
Food Processing Industries - Part 2
Hydrogen sulfide (H2S) is the most commonly known and prevalent odoriferous gas associated with wastewater treatment systems. In addition to being toxic and corrosive to delicate instrumentation and other metals, it has a characteristic rotten egg odor that is objectionable and disagreeable. In breweries, H2S odors may come from the biochemical reduction of inorganic sulfur compounds. It is generally recognized that under anaerobic conditions, sulfate-reducing bacteria (SRB) use sulfate as an oxygen source to metabolize organic matter in the waste stream.
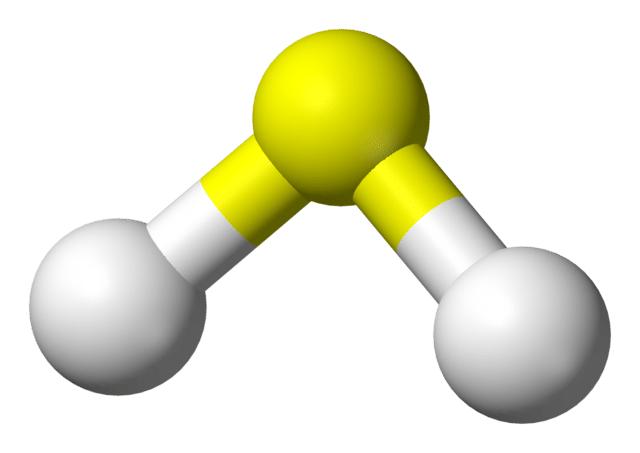
How Hydrogen Sulfide Causes Odors
Primarily, the SRB use a transport protein to bind sulfate in the bulk water. This provides the key to the sulfate entering into the periplasmic space (space outside and surrounding the cell membrane but inside the cell wall) of the bacteria where it can be further acted upon as an electron acceptor in the bacteria’s complex energy process. This sulfate – binding protein has been isolated by A. B. Pardee1 and is known to contain one binding site for sulfate per molecule. It is at the point where the water-soluble sulfate is reacted upon by the bacteria that proprietary inhibitors apparently interfere with the reaction. This means that the inhibitor either prevents the reaction from occurring or prevents subsequent reactions from going forward and producing hydrogen sulfide. The exact means or reaction of the inhibitors that causes the inhibition of the sulfate uptake or reduction is not completely understood. Studies to isolate the exact mechanism are being done.
Preliminary research suggests that there may be a quinone-like functionality in the inhibitor. This may be similar to the well-known anthraquinone mechanism of inhibition established in prior art. That is, certain cytochrome locations are involved in the SRB metabolic process. It may be that the inhibitors block the SRB’s sulfate reduction at this site, among other possibilities. Conditions favorable to the formation of H2S also increase the opportunity for the formation of other malodorous compounds such as mercaptans. At the same time, nitrogen-containing compounds such as amines may be present. These amine and ammonia compounds may also cause noxious odors that are disagreeable.

