What is the pH of DI Water?
What is the pH of DI Water?
pH Values
What is the pH of DI water? -An article appeared in the publication “American Laboratory” that established a sure-fire method of determining the minimum and maximum pH values possible at known specific resistance values. The article discusses various causes of erroneous pH measurements and concludes that from specific resistance readings one can accurately determine the pH range of the water being tested. The graph below is provided to plot minimum and maximum pH values possible at any known specific resistance value up to 18 megohms. The graph depicts the relationship.
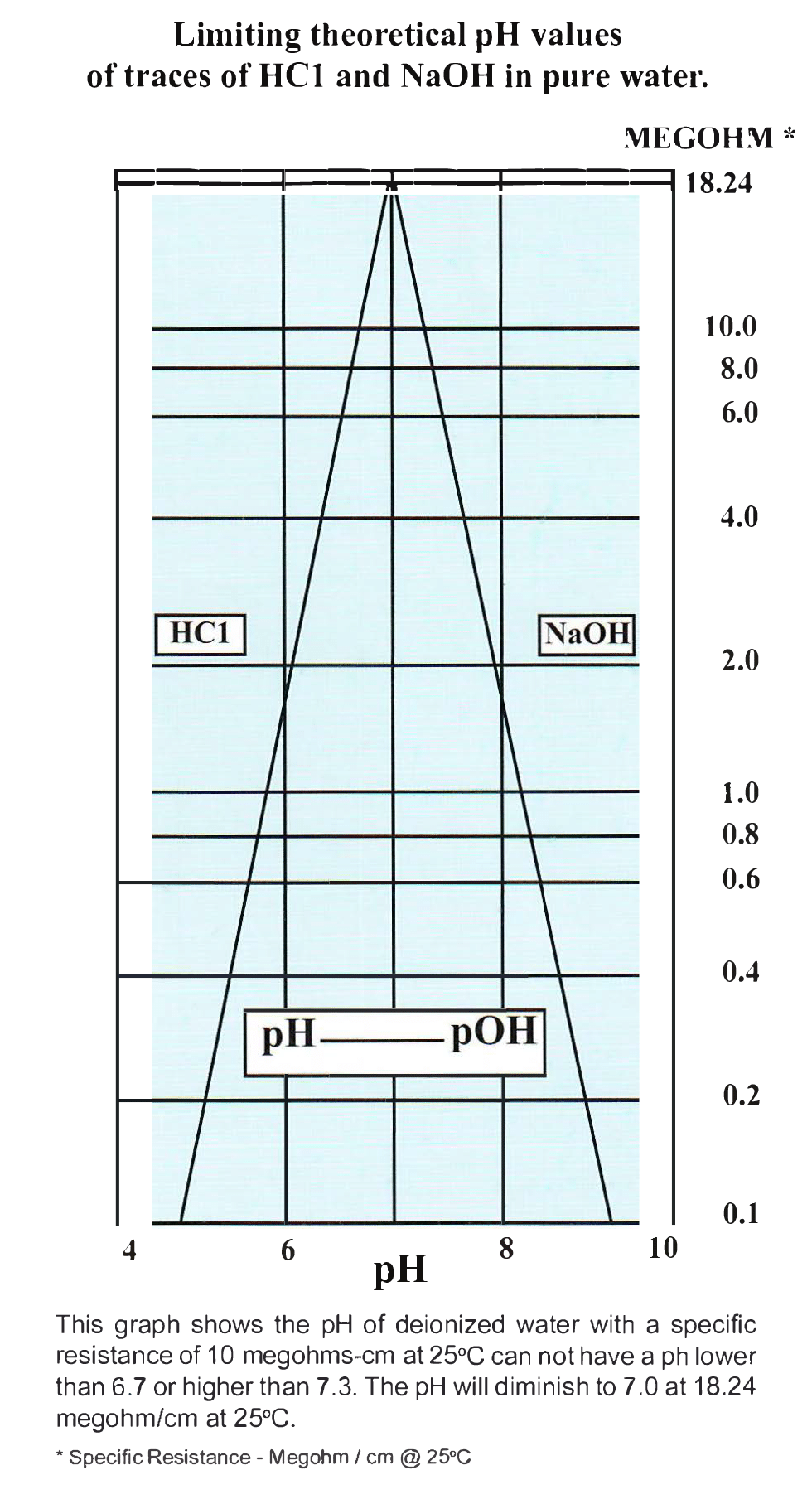
Determining pH of Water
The acidity of a water normally can be though of in terms of the concentration of hydrogen ions present in it. This can vary over a wide range of low concentrations and expressing it in a logarithmic in terms results in a reasonable range of numbers. pH is defined as -log10 hydrogen ion concentration. Thus the more acid a solution, the larger the hydrogen ion concentration and the lower the pH. Pure water contains both hydrogen and hydroxide ions at concentrations of 10·7 mol/1. It thus has a pH of 7. A more acid water has a pH of less than 7 and a more alkaline water, more than 7. The pH of water, and the ease with which this is changed by the addition of acids or alkalies, depends on the relative and total concentrations of the different substances already present in the water.
pH Test and Test Water
The pH test is one of the most widely used tests in the water industry and is also the least understood. It is generally accepted that pure water is “too pure” to indicate an ionic reaction measurable by potentiometry and the meter of a pH instrument is very unstable when the attempt is made. The use of pH papers is unreliable; values may differ from a meter by as much as 2 units. If a drop of neutral salt solution, (such as the saturated potassium chloride used in a reference electrode), is added to the test water, a completely reliable pH can be determined by potentiometry and readings are stable and reproducible. pH may be measured on any instrument used for aqueous pH measurements if the instrument is standardized against a pH 7.0 buffer just before use. A 100 milliliter sample beaker is rinsed three times with the test water and 0.30 milliliters of saturated potassium chloride is added to the solution in the beaker. The test water should be added to the beaker without agitation and the pH measured immediately.
pH of High Quality Deionized Water
Very high quality deionized water may produce a pH measurement of 6.0 to 6.4 following this test procedure. This is due to exposure to the atmosphere, absorption of gases (primarily CO2 ), the formation of carbonic acid, and the lack of buffering salts (whereby minute amounts of carbonic acid will produce a sizeable pH change). When testing very high quality DI water, it is critical that the sample have reduced exposure to the atmosphere and the test be performed immediately.

