The Principle of Reverse Osmosis and Nanofiltration
The Principle of Reverse Osmosis and Nanofiltration
How Reverse Osmosis Works
The natural phenomenon of osmosis occurs when pure water flows from a dilute saline solution through a membrane into a higher concentrated saline solution.
The phenomenon is illustrated below: A semi-permeable membrane is placed between two compartments. “Semi-permeable” means that the membrane is permeable to some species, and not permeable to others. Assume that the membrane is permeable to water, but not to salt. Then, imagine placing a salt solution in one compartment and pure water in the other compartment. The membrane will allow water to permeate through it to either side, but salt cannot pass through the membrane.
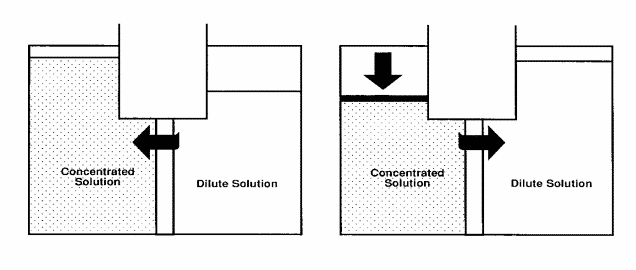
Osmosis
Water diffuses through a semi-permeable membrane toward a region of higher concentration to equalize strength. The height difference between the columns/compartments is known as the “osmotic” pressure.
Reverse Osmosis
Applied pressure in excess of osmotic pressure reverses water flow direction. Hence the term “reverse osmosis”.
As a fundamental rule of nature, this system will try to reach an equilibrium. That is, it will attempt to reach an equal concentration on both sides of the membrane. The only possible way to reach equilibrium is for water to pass from the pure water compartment to the salt-containing compartment, diluting the concentration of the salt solution.
Also visible in the illustration above, osmosis can cause a rise in the height of the salt solution. This will continue to increase until the pressure of the column (salt solution) is so high that the force of this water will stop the water flow. The equilibrium point of this water column height in terms of water pressure against the membrane is called osmotic pressure.
If a force is applied to this column of water, however, the direction of water flow through the membrane can be reversed. This is the basis of the term “reverse osmosis”. Note that, due to the semi-permeability of the membrane, the reversed flow produces pure water from the salt solution, as the membrane is not permeable to salt.
How Nanofiltration Works
Nanofiltration membranes are not a complete barrier to dissolved salts. Depending on the type of salt and the type of membrane, the salt permeability may be low or high. If the salt permeability is low, the osmotic pressure difference between the two compartments may become almost as high in reverse osmosis. However, a high salt permeability of the membrane would not allow the salt concentrations in the two compartments to remain very different. Thus, osmotic pressure plays a minor role if the salt permeability is high.
How to Use RO and Nanofiltration in Practice
In practice, reverse osmosis and nanofiltration are applied as a crossflow filtration process. The simplified process is shown in the figure below.
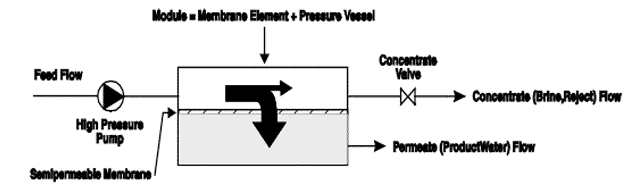
With a high pressure pump, feed water is continuously pumped at elevated pressure to the membrane system. Within the membrane system, the feed water will be split into a low-saline and/or purified product, called permeate, and a high saline or concentrated brine, called concentrate or reject. A flow regulating valve, called a concentrate valve, controls the percentage of feedwater that is going to the concentrate stream and the permeate which will be obtained from the feed.

Key Terms Used in the RO/Nanofiltration Process
Recovery – the percentage of membrane system feedwater that emerges from the system as product water or “permeate”. Membrane system design is based on expected feedwater quality and recovery is defined through initial adjustment of valves on the concentrate stream. Recovery is often fixed at the highest level that maximizes permeate flow while preventing precipitation of super-saturated salts within the membrane system.
Rejection – the percentage of solute concentration removed from system feedwater by the membrane. In reverse osmosis, high rejection of total dissolved solids (TDS) is important, while in nanofiltration, the solutes of interest are specific, e.g., low rejection for hardness and high rejection for organic matter.
Passage – the opposite of “rejection”, passage is the percentage of dissolved constituents (contaminants) in the feedwater allowed to pass through the membrane.
Permeate – the purified product water produced by a membrane system.
Flow – Feed flow is the rate of feedwater introduced to the membrane element or membrane system, usually measured in gallons per minute (gpm) or cubic meters per hour (m³/h). Concentrate flow is the rate of flow of non-permeated feedwater that exits the membrane element or membrane system. This concentrate contains most of the dissolved constituents originally carried into the element or into the system for the feed source.
Flux – the rate of permeate transported per unit of membrane area, usually measured in gallons per square foot per day (gfd) or liters per square meter an hour (L/m²h).
Factors Affecting Reverse Osmosis and Nanofiltration Performance
Permeate flux and salt rejection are the key performance parameters of a reverse osmosis or a nanofiltration process. Under specific reference conditions, flux and rejection are intrinsic properties of membrane performance. The flux and rejection of a membrane system are mainly influenced by variable parameters including:
- Pressure – With increasing effective feed pressure, the permeate TDS will decrease while the permeate flux will increase.
- Temperature – If the temperature increases and all other parameters are constant, the permeate flux and the salt passage will increase.
- Recovery – Recovery is the ratio of permeate flow to feed flow. In the case of increasing recovery, the permeate flux will decrease and stop if the salt concentration reaches a value where the osmotic pressure of the concentrate is high as the applied feed pressure. The salt rejection will drop with increasing recovery.
- Feed Water Salt Concentration – If the feed water concentration increases and all other parameters are constant, the permeate flux will decrease as well as the salt rejection.
To discuss Reverse Osmosis and Nanofiltration and how it relates to your industrial or commercial water system, contact the experts at Complete Water Solutions today. We’d be happy to help you determine the best solutions for your needs and answer any questions!

