The Benefits of EDI Water
The Benefits of EDI Water
Application of EDI for Ultrapure Water Production
Authors: Brian P. Hernon, R. Hilda Zanapalidou, Li Zhang, Linda R. Siwak, and Erik J. Shoepke
Presented at the 55th Annual Meeting International Water Conference
Pittsburgh, PA, Oct. 30-Nov. 2, 1994
Note: SUEZ purchased Ionics in 2005
Introduction
Ultrapure water is critical in a number of industrial applications, such as power generation, semiconductor manufacturing, and pharmaceutical formulation. Traditionally, ion exchange has been used to provide ultrapure water in these industries but membrane processes are becoming increasingly popular as pretreatment steps or as replacements for ion-exchange systems altogether. Membrane processes can provide very high levels of demineralization and offer the advantage of continuous operation. Moreover, membrane processes are not as mechanically complex as ion- exchange systems, and they require no acid and caustic regeneration, nor waste neutralization.
In semiconductor fabrication, the use of reverse osmosis (RO) is considered essential to achieve the purity levels necessary for wafer production. In power plants, increased recognition of the effect of ultrapure water quality on system component life has resulted in demand for membrane systems which can render an even higher purity of water than mixed-bed deionization systems alone can achieve (e.g. lower TOC, particles, etc.). Such high levels of demineralization can be obtained using electrodeionization (EDI). EDI combines ion-exchange resins, ion-exchange membranes, and a direct (DC) electric field. Basically, EDI is an electrodialysis (ED) process modified by the addition of ion-exchange resin. When EDI is used for the production of high purity water, the ion-exchange resin beads enhance mass transfer, facilitate water splitting, and reduce stack resistance. The ion-exchange resin exchanges ions with the incoming feed stream. The DC electrical field splits water into hydrogen and hydroxyl ions, which, in turn, continuously regenerate the ion- exchange resins. The exchanged ions are transferred through the membranes to the brine stream and flushed from the system.1,2 Compared to conventional ion exchange, EDI has the advantage of being a continuous process with constant stable product quality, which is able to produce ultra-high purity water without the need for acid or caustic regeneration.
This paper presents data from five commercial-scale EDI installations now in operation. Four of these EDI installations are located at electric generation plants. The fifth EDI system is part of a silicon wafer manufacturing facility located in the Midwest (Si FAB). The electric power plants are Grand Gulf Nuclear Station (GGNS), Arkansas Nuclear One (ANO), Florida Power & Light Turkey Point Power Station (TPPS), and the Riverbend Nuclear Station (RBNS). In the power plants, the ultrapure water is used for makeup to high-pressure boilers and for plant usage. In the semiconductor plant, ultrapure water is used for wafer rinsing.
In each of the five installations discussed in this paper, the EDI units are integral parts of multi-step purification systems that supply high purity water. The EDI process pretreatment includes filtration and partial demineralization by reverse osmosis (RO). RO removes many of the contaminants which could harm an EDI system, such as organics, which can foul anion and cation ion-exchange resins, and particulate matter, which would be difficult to remove from an EDI unit. Scaling ions, such as magnesium and calcium which, at high enough concentrations, could necessitate the use of continuous acid addition to the EDI, are removed at least 95 percent by an RO system. The combination of reverse osmosis and EDI allows the EDI to perform at its optimum level. In a process train in which RO is followed by EDI, water can be produced that is comparable to mixed-bed ion-exchange treated water.3
Performance
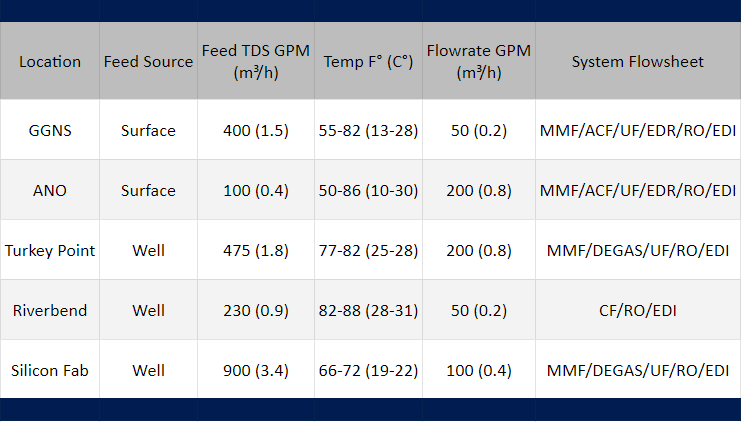
In the installations reported in this paper, EDI is part of water treatment processes that deal with quite different feedwater conditions. The processes vary in feedwater sources, composition, and pretreatment equipment upstream of the RO unit. In all cases, the water treatment systems also have to handle feed water quality variations, such as seasonal changes in the ionic load, temperature, organics and pH. Such changes, as well as the process conditions, affect the performance of the EDI demineralizers. Table 1 lists the unit processes that are used in each installation and summarizes key variables describing the feedwater.
In all of the cases in Table 1, the EDI product water is further polished using portable ion exchange beds. Portable ion-exchange beds are economical to use in these cases since the EDI product water has a very low ionic load which translates into long bed life, usually several months in length. Portable ion- exchange beds also offer higher water quality than is normally achieved in conventional in-situ ion- exchange systems, since resin regenerations are conducted in small volumes at one dedicated facility where regeneration of resins can be tightly monitored and controlled.