The Adsorption Process
The Adsorption Process
The Adsorption Process
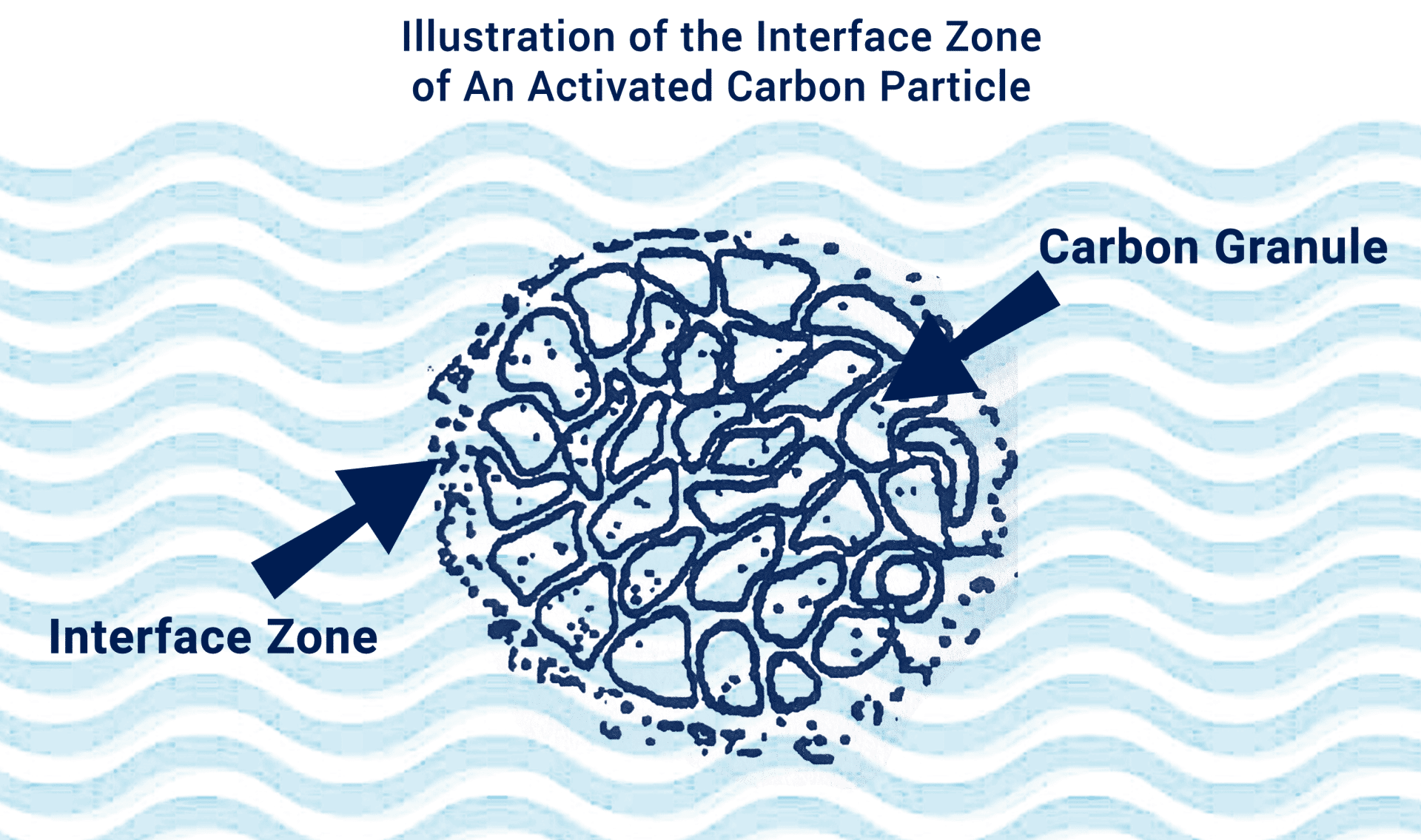
Adsorption is the phenomenon which occurs when various substances accumulate at the “interface” zone (see image below) between two phases: a liquid and a solid or a gas and a solid. These absorbates are held on the surface of various adsorbent products by physical and chemical forces. The major absorbents used in water treatment for homes, businesses, and farms include granular activated carbons, anion exchange resins, adsorbent resins, and activated alumina.
Adsorption has been practiced throughout the world for centuries. The ancient Egyptians, Indians, and Persians put charred wood in beverages to improve flavor. According to records compiled by Windle-Taylor, filtering water through charcoal (in Sanskrit lore) to improve it’s quality dates back to the year 2000 BC. Purification by adsorption is on the oldest clarification processes. For generations the adsorption process has been used to remove organic substances and to clarify beer and wine. Sugar refining has used adsorption to produce white, crystalline sugars. Important present day non-liquid uses of adsorption principles include air filters, gas masks, and organic solvent separation.
In a more simplified term, adsorption can be thought of as an invisible, magnetic-type field surrounding a particle of adsorbent product. As Michaud has said, all molecules attract one another. A granular, clean, activated carbon surface, has strong attraction forces for what are called nonpolar materials. When activated carbon forces are greater than the forces that keep these materials in solution (water), then the materials will adsorb, or adhere, to the activated carbon surface.
The Mechanics of Adsorption
When interaction results in the distribution of one phase within the bulk substance of another, the reaction that takes place is absorption. The adsorption process limits this interaction of the liquid and solid substance to the interface zone, the finite region between the two. Hence, adsorption is a surface of an adsorbent medium. This process is a useful tool in separating species and providing a means of recovering valuable materials in several industrial processes.
Adsorption occurs when the surface energy of a solid (adsorbent) substance attracts a molecular specie from a liquid. These weak forces, called Van der Waals forces, are the same forces that cause the phenomena of surface tension. When the Van der Waals forces are exclusively responsible for the adsorption, the process is called “physical adsorption,” and it can be easily reversed by the step known as “desorption.”
When the forces that attract the substance to the surface of the adsorbent are derived from the chemical ionic nature of the adsorbent’s surface, the process is known as chemisorption and is generally not reversible.

Absorption vs. Adsorption
Absorption differs from adsorption. The two terms are frequently used incorrectly. Absorption means to “drink in, like a sponge imbibes water” which is the process of assimilation of molecules into a solid. Adsorption concerns those processes by which matter adheres to the surface areas of a solid adsorbent material. Both absorption and adsorption processes illustrate certain phenomena that tend to be more physical than those of water chemistry. They involve a solid surface medium and a liquid water solution.

