How to Identify Water Impurities
How to Identify Water Impurities
The impact of the various physical impurities of water generated during the hydrologic cycle and/or bacterial colonization depends upon the water user’s particular requirements. In order to assess the need for treatment and the appropriate technology, the specific contaminants must be identified and measured.

Qualitative Identification
Qualitative identification is usually used to describe the visible or aesthetic characteristics of water. Among others these include:
• turbidity (clarity)
• taste
• color
• odor
Turbidity - Suspended Impurities in Water
Turbidity consists of suspended material in water, causing a cloudy appearance. This cloudy appearance is caused by the scattering and absorption of light by these particles. The suspended matter may be inorganic or organic. Generally the small size of the particles prevents rapid settling of the material and the water must be treated to reduce its turbidity.
Correlation of turbidity with the concentration of particles present is difficult since the light-scattering properties vary among materials and are not necessarily proportional to their concentration. Turbidity can be measured by different optical systems. Such measurements simply show the relative resistance to light transmittance, not an absolute level of contamination.
Suspended matter can also be expressed quantitatively in parts per million (ppm) by weight or milligrams per liter (mg/L). This is accomplished by gravimetric analysis, typically filtering the sample through a 0.45-micron membrane disc, then drying and weighing the residue. The Silt Density Index (SDI) provides a relative value of suspended matter. The measured values reflect the rate at which a 0.45-micron filter will plug with particulate material in the source water. The SDI test is commonly used to correlate the level of suspended solids in feedwater that tends to foul reverse osmosis systems.
Taste
The taste sense is moderately accurate and able to detect concentrations from a few tenths to several hundred ppm. However, taste often cannot identify particular contaminants. A bad taste may be an indication of harmful contamination in drinking water, but certainly cannot be relied on to detect all harmful contaminants.
Color
Color is contributed primarily by organic material, although some metal ions may also tint water. While not typically a health concern,
color does indicate a certain level of impurities, and can be an aesthetic concern. “True color” refers to the color of a sample with its turbidity removed. Turbidity contributes to “apparent” color. Color can be measured by visual comparison of samples with calibrated glass ampules or known concentrations of colored solutions. Color can also be measured using a spectrophotometer.
Odor
The human nose is the most sensitive odor-detecting device available. It can detect odors in low concentrations down to parts per billion (ppb). Smell is useful because it provides an early indication of contamination which could be hazardous or at least reduce the aesthetic quality of the water.
Further Analysis - Dissolved Impurities in Water
Further analysis should focus on identification and quantification of specific contaminants responsible for the water quality. Such contaminants can be divided into two groups: dissolved contaminants and particulate matter. Dissolved contaminants are mostly ionic atoms or a group of atoms carrying an electric charge. They are usually associated with water quality and health concerns. Particulate matter – typically silt, sand, virus, bacteria or color-causing particles – is not dissolved in water. Particulate matter is usually responsible for aesthetic characteristics such as color, or parameters such as turbidity, which affects water processes.
Quantitative Identification
The following are the major quantitative analyses which define water quality.
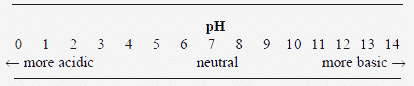
pH
The relative acidic or basic level of a solution is measured by pH. The pH is a measure of hydrogen ion concentration in water, specifically the negative logarithm (log) of the hydrogen ion concentration. The measurement of pH lies on a scale of 0 to 14 (Figure 2), with a pH of 7.0 being neutral (i.e., neither acidic nor basic), and bearing equal numbers of hydroxyl (OH-) and hydrogen (H+) ions. A pH of less than 7.0 is acidic; a pH of more than 7.0 is basic.
Since pH is expressed in log form, a pH of 6.0 is 10 times more acidic than a pH of 7.0, and a pH of 5.0 is 100 times more acidic than a pH of 7.0. The pH has an effect on many phases of water treatment such as coagulation, chlorination and water softening. It also affects the scaling-potential of water sources. The pH level can be determined by various means such as color indicators, pH paper or pH meters. A pH meter is the most common and accurate means used to measure pH.
Total Solids
Total Solids (TS) is the sum of Total Dissolved Solids (TDS) and Total Suspended Solids (TSS). In water analysis these quantities are determined gravimetrically by drying a sample and weighing the residue. In the field, TDS is commonly measured by a conductivity meter which is correlative to a specific salt solution; however, this measurement is only an approximation most often based on a multiplication factor of 0.66 of the electrical conductivity.

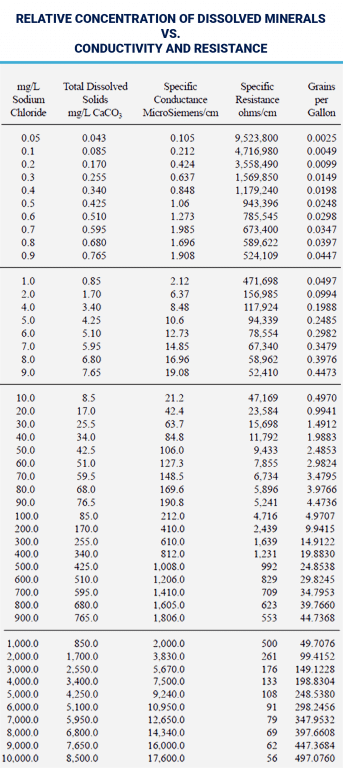
Conductivity/Resistivity
Ions conduct electricity. Because pure water contains few ions, it has a high resistance to electrical current. The measurement of water’s electrical conductivity, or resistivity, can provide an assessment of total ionic concentration. Conductivity is described in microSiemens/cm (μS) and is measured by a conductivity meter and cell. Resistivity is described in megohm-cm, is the inverse of conductivity and is measured by a resistivity meter and cell.
The table below expresses the relative concentrations of sodium chloride versus conductivity and resistance. As a general rule, ionic-dissolved
content, expressed in ppm or mg/L, is approximately one-half to two-thirds the conductance of water. Other salt solutions are used and the curve varies. Monovalent salts have higher conductivities than multivalent salts.