How Water Softener Exchangers Work
How Water Softener Exchangers Work
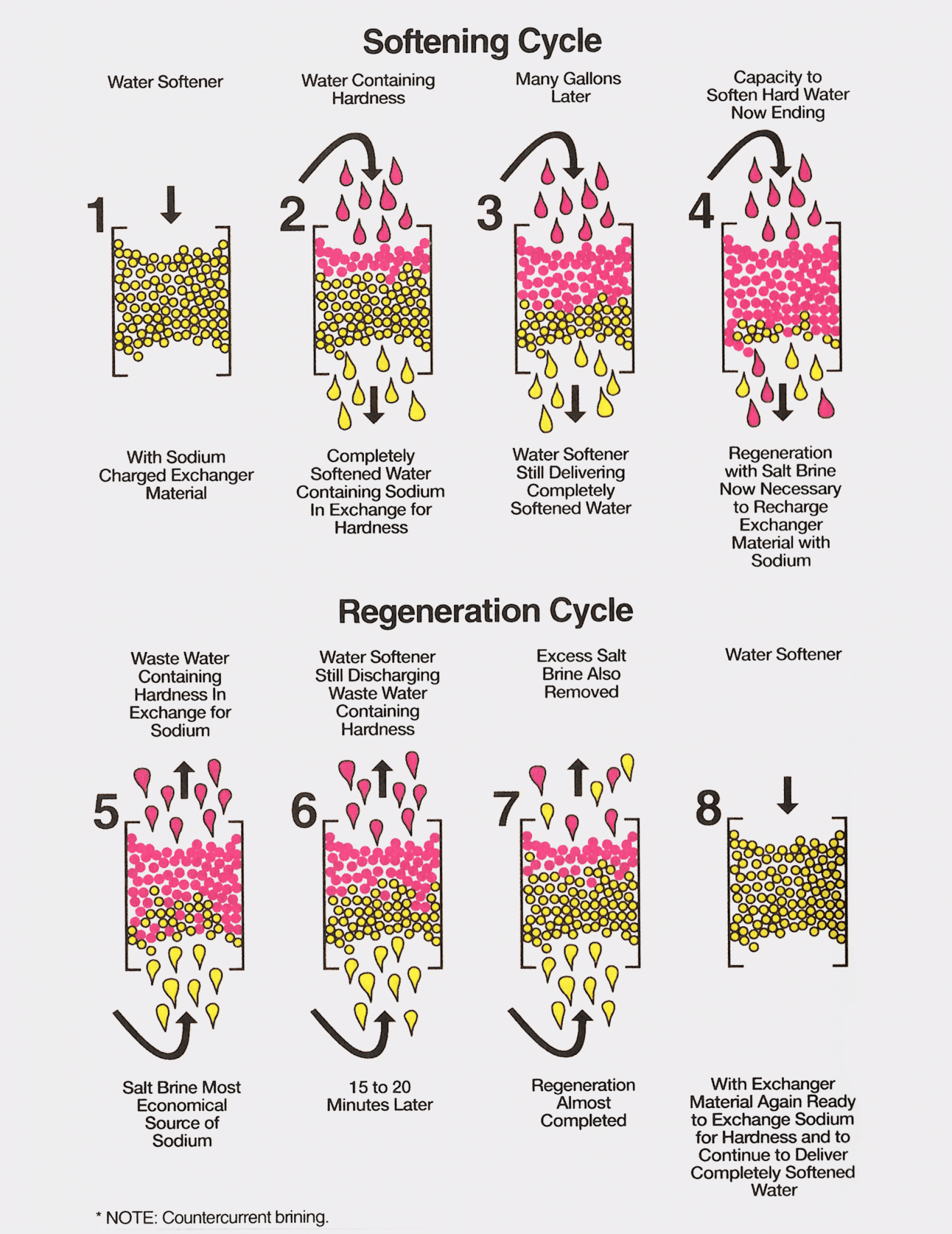
The softening of water by the cation exchange process relies on the replacement of calcium and magnesium ions for a chemically equivalent number of sodium ions or potassium ions, depending on which regenerant solution is used. The softening cycle and the regeneration process of cation exchanger softeners are graphically illustrated in the image below.
Conventional ion exchange products are tiny, insoluble plastic beads of polystyrene cross-linked with divinylbenzene measuring from 0.4 to 1.2 millimeters in size. Standard softening ion exchangers typically contain 45-47 percent moisture (water). The exchanger beads can be visualized as skeleton-like structures having many attached “exchange sites”. (See image at right)
The skeleton is insoluble in water but is electrically charged, holding ions of opposite charge to the species to be removed at the exchange sites.
Ion exchange resins with negatively charged (-) skeletons are referred to as cation exchangers, since they attract positive (+) ions called “cations.” If positive cations such as calcium, iron, and manganese or cation species (other than sodium or potassium) should approach a cation exchanger particle saturated with sodium or potassium, some of them can be captured by the exchanger. This chemical reaction, in turn, would release the sodium or potassium ions previously held at the exchanger sites on the skeleton beads. An equilibrium is soon established between the distribution of these species on the exchanger and in the water surrounding the exchanger beads.
The softening of water by the ion exchange process relies on the replacement of calcium and magnesium ions for a chemically equal number of either sodium or potassium ions for a chemically equal number of either sodium or potassium ions, depending on the regenerant. The process may be likened to the “swapping of ions”, or to the operation of a vending machine. Chemically the process occurs as expressed in the following equation:
| 2RNA | + | Ca(HCO₃)₂ | → | R₂Ca | + | 2NaHCO₃ |
|---|---|---|---|---|---|---|
| sodium ion exchange resin | calcium bicarbonate in water | calcium ion exchange resin | sodium bicarbonate in water |
Regeneration of the resin back to the sodium form is usually done by passing a solution of sodium chloride (NaCl) brine through the resin bed. The reaction is illustrated by this equation:
| R₂Ca | + | 2NaCl | → | 2RNa | + | CaCl₂ |
|---|---|---|---|---|---|---|
| calcium ion exchange resin | sodium chloride (brine) | sodium ion exchange resin | calcium chloride in water going to waste |