Aeration Processes
Aeration Processes
The Aeration Process
Water aeration has been taking place in nature since the beginning of time. Typical examples include the action of waterfalls such as Niagara Falls at the Canada-New York border and the swift-flowing white-water rapids of the Colorado River. Both of these examples of natural aeration permit water to absorb oxygen from the atmosphere, thereby making the survival of many fish possible. On the other hand, as the great geysers in such places such as Yellowstone Park spew out their mists into the atmosphere, some dissolved gases are released. Thus, the aeration process can serve two important functions in water treatment: (1) degasification or (2) oxygenation. After filtration, the aeration process is perhaps the second oldest treatment technology in use today. Its functions are quite readily understood, and the results of this treatment process can be easily observed. At dairies, milk is sometimes aerated to remove barn odors by running the milk overexposed, chilled surfaces immediately after milking.
In water treatment, the term “aeration” is applied to those processes in which: 1. water is brought into intimate contact with air, or 2. air is brought into intimate contact with water, for the purpose of changing the concentrations of volatile substances contained in the source water. (Note: “air stripping” is a form of aeration.)
Aeration of water in combination with exposure to sunlight was one of the earliest water purification methods employed. The oxygen absorbed through aeration and the effect of the natural ultraviolet rays emitted by the sun constituted fairly effective disinfection of the aerated water supply. The open-gravity aeration process has long been used for certain water quality conditions in large-scale water processing, such as municipal and industrial applications, at comparatively low costs.
The fundamental purpose of water aeration is to improve both the physical and chemical characteristics of a water supply. In doing so, an aeration system can, in some cases, decrease concentrations of volatile substances, and in other cases, increase volatile substances. The aeration of water is practical for:
- The mechanical reduction of dissolved residual gases, volatile synthetic organic chemicals, and hydrocarbons; and removal of some objectionable odors by reducing odoriferous matter; and
- The chemical reduction of ferrous iron and manganous manganese.
Types of Aeration Systems
Two basic categories of water aeration systems are used in industrial/municipal applications, and both are employed for business and industrial aeration as well. They are:
- Open-gravity systems in which aerated water must be repumped to repressurize the service water, and
- Closed-pressure systems that do not require repumping to repressurize the water system itself.
Open-Gravity Aerators
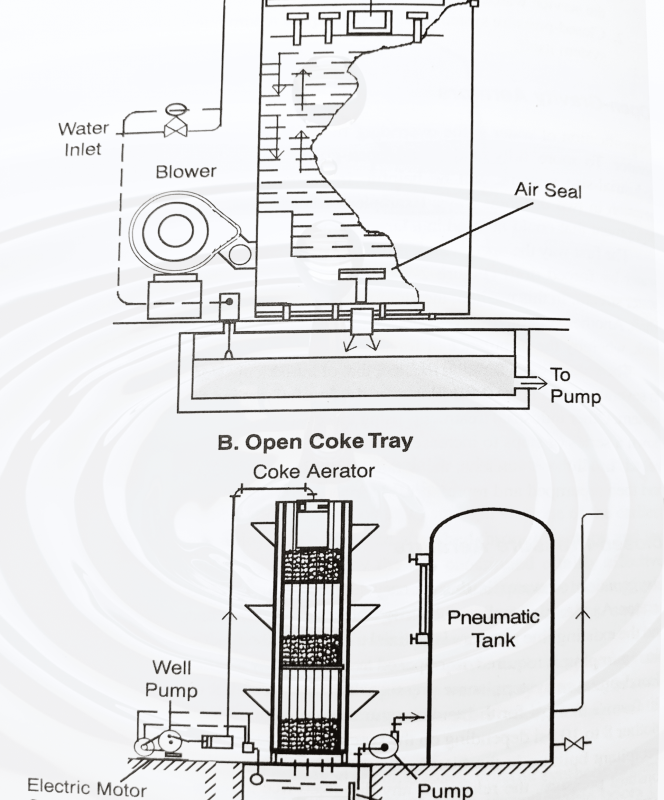
Degasification of water is the overriding function and application of the open-gravity aerator. To more fully understand open-gravity technology, a brief look at large, industrial-size systems will be helpful. In principle, the open system accomplishes aeration in two different ways. Examples of both are shown in the image below, typical of equipment that is commonly used in large municipal or industrial water treatment plants.
The first way the open aerator is used involves bringing air into intimate contact with water by forced draft, blowing air in countercurrent to the dispersed water stream. In this method, the air along with escaping substances exits through the air shaft. Another version involves bubbling compressed air upward through water in a large tank, whereby the turbulence of forced air carries off volatile substances.
The second open aeration method, that of introducing water to air, can be carried out in several ways. For instance, in the image below, the free-fall method through a coke tray aeration system is illustrated. Other industrial treatments using this concept include waterfalls, cascading water down a series of steps to increase surface exposure, and just spraying water into a large vessel. In all of these versions, the aerated water must be collected in some form of basin and then repumped and repressurized for service water.
Closed-Pressure Aerators
Oxygenation of water is the primary function and application of the closed-pressure aerator. As the title implies, the closed-pressure aerator is under constrant static pressure, and the existing line pressure is utilized to carry out the functions involved in this process. No repumping is required, as opposed to open-gravity aeration methods. In operating the closed-pressure system, some pressure drop can occur, but usually this is minor and can run from 2 to 5 psi for the aerator-saturator itself. Any subsequent filtering step will add another 8 to 10 psi depending on the type of filter medium, control valve design, and any precipitant built up in the filter between backwashing cycles. Since the system is under constant pressure, the release of any dissolved free gases or other volatile substance is extremely limited. Only through the blowoff by a pressure relief device can any volatile substances be exited from the closed aerator system.
In the closed-pressure units, air is introduced to water somewhat in the same fashion described in the section of open-gravity systems using forced draft. One closed-pressure industrial-type method is illustrated below and shows the system bringing air into intimate contact with water. In this scheme, only a fraction of the water enters an air-saturator, where the necessary air is supplied by an air compressor, which in turn is regulated by a level controller. This predetermined portion of water enters the top of the saturator through baffling and rains downward through the air saturation zone to the bottom of the tank, where it exits into and mixes with the main flow. This oxygenation process is dependent on the contact time of the water within the air-saturator zone, and best results are obtained by using flow controller orifices to control the flow.
A second method of introducing air into water is through the use of eductors or venturi tubes. These cause a slight pressure differential, which allows air to be sucked in and mixed with the water stream.
The closed-pressure aerator systems are best suited for the oxygenation of water from chemical reactions, which can transform dissolved metal substances into metal hydroxide compounds. The hydroxides, being less soluble in water, will “drop out” as precipitants that can then be filtered out of the stream.