How is Water Used in Pretreatment Systems?
How is Water Used in Pretreatment Systems?
The powder coating industry uses a variety of pretreatment methods to prepare metal parts. Water is used in parts washers, together with pretreatment chemicals and conversion coatings. The chemical suppliers offer a wide variety of aqueous cleaner, primarily to remove organic and inorganic soils (oil, grease, polymers, soaps, oxides, and particulates) from metal parts so that the powder coatings can mechanically bond to the substrate. In addition, iron or zinc phosphate conversion coatings promote powder adhesion, enhance corrosion risesistance, and limit flash rust.
Each chemical system has its own merits for a specific application. Much time and effort is spent by plant personnel in the powder coating industry to select chemical treatments and to monitor a spray washer for temperature, pH, titration, TDS (Total Dissolved Solids), conductivity, and general washer conditions such as spray-nozzle condition, placement and pressures. Each of these variables will affect coating performance. Good water quality is essential to the pretreatment system. Any naturally occuring contaminants in the source water such as mineral hardness, dissolved salts, high alkalinity, chlorine, and so on can limit the quality of the coating. When calcium and magnesium from source water come into contact with phosphates, silicates, and carbonates, there are more than 50 different insoluble salts that will precipitate out of solution (in the heated stages of a washer) forming sludge buildup and scale on heat exchangers.
Plant personnel in the powder coating industry must realize that these dissolved solids in the water will be carried to and deposited on metal parts while rinsing. The same is true for the final rinse water. Even if some form of acidified sealer is used, when the final water droplets evaporate, the leave behind a scale (or spot) on the surface. Chlorides, sulfates, and calcium an magnesium salts remain on the metal substrate, along with other constituents present in the source water. Now the coating on top of a scale spot will actually act as a semi-permeable membrane and allow moisture to migrate through the polymer film. When the mechanical adhesion of the coating to the substrate is poor, moisture will actually permeate the coating film and cause the coating to swell, which will create a corrosion site and lift the coating film away from the substrate. In addition, this transmitted moisture can now redissolve salts and continue to break away the swelled area. This effect can be significant with high TDS source water used for final rinse.
How Do You Create Spot-Free Rinse Water for Your Washer?
As electrolytes in the form of dissolved salts are removed from water, the water’s ability to conduct electricity decreases. This causes an increase in the water’s resistance measured in ohms. Spot-free final rinse water for typical finishing lines must have a resistivity greater than 50,000 ohms. This corresponds to a TDS level of less than 8 parts per million (ppm). Reference Table 1, which shows specific conductance and the corresponding approximate electrolyte concentration in ppm (salt).
For final rinse water to be spot-free, it must be in the range of less than 10mg/L of TDS. To create spot-free rinse water, many companies must treat their source water by running it through a deionizer system. This equipment was the best available technology years ago as the powder coating industry was first emerging from its infancy. A deionizer will remove more than 99 percent of the source water TDS. However, today, many new and retrofit washers are being designed with reverse osmosis (RO) membrane systems. The RO process willl reduce the source water TDS by approximately 98 percent and is much more environmentally friendly and less costly to operate than a deionizer system. In many instances, a 98 percent reduction of source water TDS will get you into that spot-free rinse range of less than to mg/L TDS. If not, additional equipment may be needed for certain feedwater supplies.
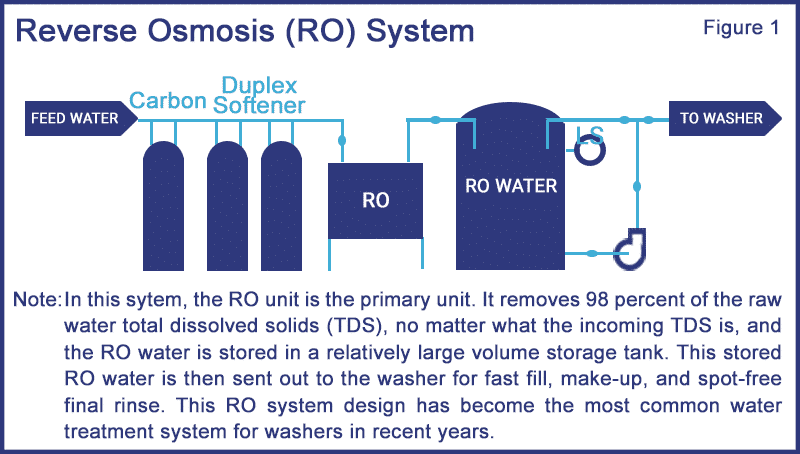
To calculate the approximate TDS content of the purified water produced by an RO machine, simply multiply the source water TDS content by 0.02 (two percent remaining). Refer to Figure 1, which shows a typical RO sytem. There are actually four different filtering processes taking place here:
- The carbon filter absorbs chlorine from the feed water.
- The water softener removes calcium and magnesium mineral hardness from the feed water.
- An inlet prefilter rated for 1.0 micron (one millionth of a meter particle size) physically filters out colloidal sand and silt particles.
- The RO membranes reduce the TDS by 98 percent whereby the water passes through the membrane to the other side; yet the larger salt and metals are too large to pass and they remain in the drain flow from the RO machine.
Table 1
Specific Conductivity and Resistance, and Approximate Electrolyte Content
| Specific Conductivity (micromhos) | Specific Resistance (ohms) | Approx. Electrolyte Content (parts per million sodium chloride) |
| 0.1 | 10,000,000 | 0.04 |
| 0.2 | 5,000,000 | 0.08 |
| 1 | 1,000,000 | 0.4 |
| 2 | 500,000 | 0.8 |
| 4 | 250,000 | 1.6 |
| 6 | 166,000 | 2.5 |
| 8 | 125,000 | 3.2 |
| 10 | 100,000 | 4 |
| 20 | 50,000 | 8 |
| 30 | 33,333 | 14 |
| 40 | 25,000 | 19 |
| 50 | 20,000 | 24 |
| 60 | 16,666 | 28 |
| 70 | 14,286 | 33 |
| 80 | 12,500 | 38 |
| 90 | 11,111 | 43 |
| 100 | 10,000 | 50 |
| 200 | 5,000 | 100 |